How Do Water Softeners Work?

How Do Water Softeners Work?
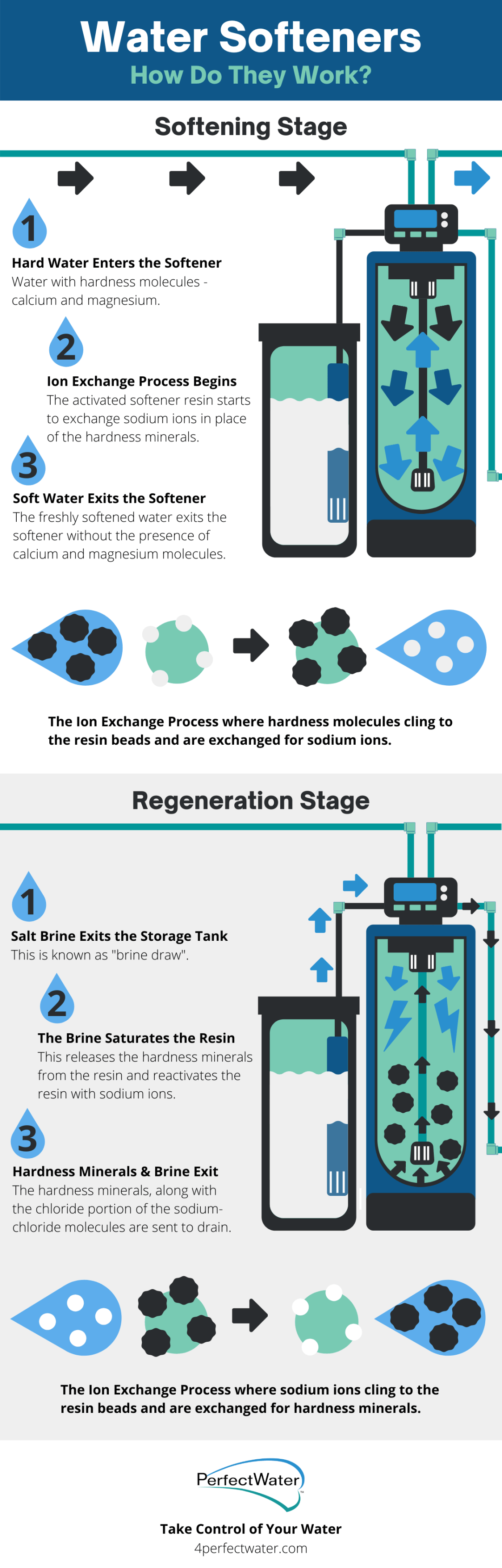
Here’s a step-by-step of how water softeners work:
- Hard water (water containing calcium and magnesium molecules) enters the water softener.
- The resin beads within the softener are positively charged with sodium molecules clinging onto the resin beads.
- When the calcium and magnesium molecules pass through the resin beads, the calcium and magnesium take the place of the sodium.
- This causes the calcium and magnesium molecules to cling to the beads and the product water includes amounts of sodium instead.
- After an adequate amount of water has gone through the system, a backwashing cycle takes place.
- This is where the myth that salt is in soft water comes into play. During the backwash cycle, sodium chloride (table salt) passes through the resin beads. It is used to wash away the calcium, magnesium and here is where it splits. The chloride gets washed away with the calcium and the magnesium and only sodium is left.
- The sodium only is left on the resin beads and the process starts all over again.
Ready to Take Control of Your Water Supply?
Don’t wait for water shortages or unreliable sources to disrupt your life. With our Rainwater Harvesting systems, you can enjoy a sustainable, reliable, and completely self-sufficient water solution tailored to your home’s needs.

