6 Myths of Water Softeners Debunked

Have you ever thought about getting a water softener but then been turned away by something negative you heard? More times than not, the information you are hearing is either incomplete or false entirely. This article takes a look at those most common myths standing in your way to softer water.
What is soft water? What is hard water?
All freshwater could be classified into two categories, soft water or hard water. The defining factor is water that contains calcium and magnesium is classified as hard water, and water that doesn’t is labeled as soft water. There are a few other contaminants that can make water “hard” but they are quite rare.
Why does it matter?
Think about all the ways you use water throughout your day. Whether you notice it or not, hard water has negative effects on almost everything it touches.
Hard water leaves a film on top of glass, metal, and porcelain surfaces. The whitish stain you continually have to clean off your shower door is a product of hard water. It also affects your water using appliances, coffee makers, ice machines, clothes washing and dishwashing machines. The inner-workings that involve moving water often get clogged and are more liable to break down when using hard water.
Water heaters, tank and tankless, are also affected by hard water. The heating coils become coated in calcium and magnesium and the efficiency reduces dramatically in just a couple of years.
There are a lot of benefits to soft water and negative effects of hard water.
Myths of Water Softeners and Soft Water
Myth #1 – There is Salt in Soft Water
This is a very common misunderstanding of soft water. It derives from the ion-exchange process that takes place to produce soft water. Here’s a quick overview of how softeners work. You’ll see how people got this idea. But if you pay close attention to the end process you’ll realize how false this myth is:
- Hard water (water containing calcium and magnesium molecules) enters the water softener.
- The resin beads within the softener are positively charged with sodium molecules clinging onto the resin beads.
- When the calcium and magnesium molecules pass through the resin beads, the calcium and magnesium take the place of the sodium.
- This causes the calcium and magnesium molecules to cling to the beads and the product water includes amounts of sodium instead.
- After an adequate amount of water has gone through the system, a backwashing cycle takes place.
- This is where the myth comes into play. During the backwash cycle, sodium chloride (table salt) passes through the resin beads. It is used to wash away the calcium, magnesium and here is where it splits. The chloride gets washed away with the calcium and the magnesium and only sodium is left.
- The sodium only is left on the resin beads and the process starts all over again.
So, while sodium is in soft water, salt (sodium chloride) is not. Soft water does NOT have a salty taste or anything of the like, because SALT is not in soft water.
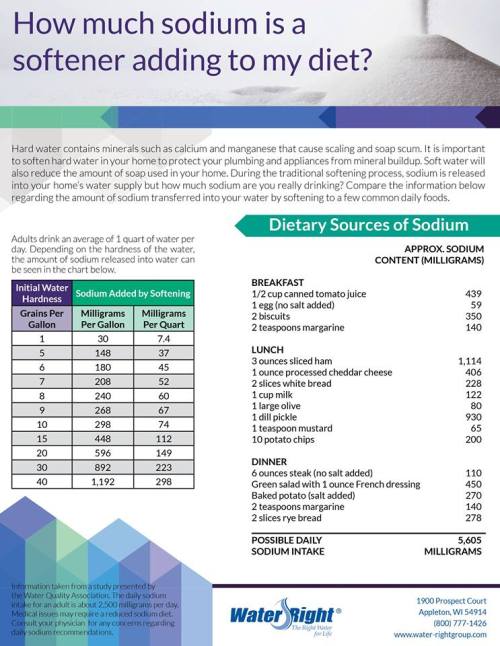
Myth #2 – The Amount of Sodium in Soft Water is Unhealthy
“The amounts of sodium in softened water are minuscule compared to other normal dietary sources of sodium. In fact, ion exchange softening of water with very high levels of hardness such as 75 grains per gallon of total water hardness would add less sodium to the drinking water than is allowed in beverages meeting the U.S. Food and Drug Administration regulations for “Low Sodium” labeling.” – Water Quality Association
In the Knoxville, TN and Nashville, TN area, it is very rare for hard water to exceed even 30 grains per gallon, which would equate to even less sodium than stated above.
*If someone is on a sodium restrictive diet, they (like everyone else) should have a reverse osmosis system. Municipal water can have sodium as well.
Myth #3 – Salt-Free Water Softeners are a Good Option
These systems do not remove calcium and magnesium. The technology used claims to eliminate scale buildup. What has been discovered is that these systems must have very exact situations for them to work properly.
There is no such thing as a salt-free softener. Softeners by definition remove calcium and magnesium, the so-called “salt-free” softeners only suspend calcium and magnesium temporarily.
To supply truly soft water at a constant flow rate, salt-using water softeners are currently the best option. (Also Rainwater Harvesting)
Here is an article that we wrote that goes into this issue on a much deeper level.
Myth #4 – Soft Water Doesn’t Rinse off Soap
When someone is used to to the feeling of soap “coming off” their skin when using hard water, the feeling of soap truly coming off when using soft water can cause one to question whether the soap is actually be removed because of the slick feeling that comes with soft water.
In reality, a layer of insoluble soap curds cover one’s skin when using hard water, that is what gives the illusion of the soap “coming off”, therefore, the slick feeling one experiences when using soft water is the is the true feeling of skin and the body’s natural oils.
Myth #5 – There is No Need for Water Softeners on Municipal (City) Water
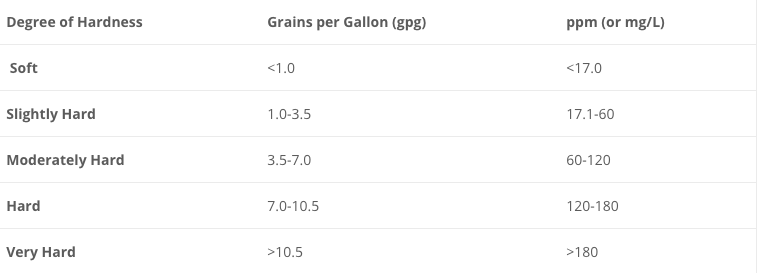
Most water municipalities are providing hard water. In Tennessee, 95% of the municipalities that we have dealt with provide water that is hard enough that the effects are obvious. The average hardness in Knoxville and Nashville, Tennessee is around 10 or 15 grains per gallon.
The degree of hardness standard as established by the American Society of Agricultural Engineers (S-339) and the Water Quality Association (WQA) is:
Symptoms include:
- Stiff, dingy laundry
- Mineral deposits on dishes and glassware
- High soap usage & need for fabric softeners
- Dry, itchy skin and scalp
- Unmanageable hair
- Extra work to remove soap curd on bathtubs & shower stalls
- High energy costs, possibly due to scale build-up in pipes and on appliances
- Scale build-up in sinks, tubs, faucets & appliances
Myth #6 – They are a Waste of Energy
Water softeners provide benefits for any machine that comes in contact with water.
The calcium and magnesium molecules in hard water cause scaling. This is true in pipes, faucets, and water-using appliances, such as dishwashers, clothes machines, and coffee makers. This scaling will often contribute to a loss in efficiency and breakdowns in appliances. Such as layering on top of heating coils in coffee maker, or clogging holes and water passages in machines like dishwashers, and can often be attributed to other breakdowns.
For water heaters (tank and tankless) a 2009 study commissioned by the Water Quality Research Foundation (WQRF) and conducted by the Battelle Memorial Institute covered this very issue. The study discovered that the water heater units maintained their factory efficiency rating for as long as 15 years when using soft water. While the unit’s efficiency would be cut as much as 48% when using hard water.
Scale buildup shortened the lifespan of the heating elements inside electric water heaters, and some tankless water heaters using hard water failed after just 1.6 years.
There are a lot of misunderstood or misleading information out there regarding soft water and hard water. Learn more about soft water and how to get it by contacting our team!
Want to learn even more about whole-home water purification in Knoxville and Nashville? Check out our Knoxville and Nashville Residents Guide to Water Purification.
Ready to Take Control of Your Water Supply?
Don’t wait for water shortages or unreliable sources to disrupt your life. With our Rainwater Harvesting systems, you can enjoy a sustainable, reliable, and completely self-sufficient water solution tailored to your home’s needs.

